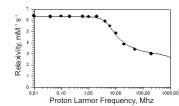
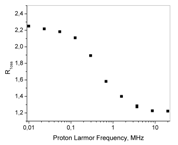
Nuclear Magnetic Relaxation Dispersion (NMRD) profile
The mobility of water molecules can be investigated by the measurement of NMR relaxation times (the spin-lattice T1 and the spin-spin T2 relaxation times ) of water protons.
The relaxation times report about the modulation of magnetic interactions of water protons with the surrounding environment and are therefore dependent on the molecular reorientational time of water molecules. Often, in biological specimens, the effective reorientational time is related to the extent of the association water molecules have with immobilized or slowly moving macromolecules. In general, the larger the macromolecule content the larger the reorientational time felt by the water protons. The relationship between the relaxation time and the dynamics of the magnetic interactions is dependent on the frequency of observation (i.e. on the applied magnetic field as w=ωB0).
The field dependence of T1 by B0 is represented in the Nuclear Magnetic Resonance Dispersion (NMRD) profiles that report about the changes in (1/T1) on function the applied magnetic field strength. A dedicated Field Cycling NMR relaxometer allows to obtain relaxation data over an extended range of Larmor Frequencies (from 0.01 to 20 MHz). Additional relaxation data can be acquired on instruments operating at higher field strength. The relaxation times T1,2 are strongly affected by the presence of paramagnetic species. In the presence of paramagnetic solutes, the observed water proton relaxation rates, (1/Ti)obs, are the sum of two contributions :
(1/Ti)obs= (1/Ti)d + (1/Ti)p i=1,2
where (1/Ti)d is the (diamagnetic) water relaxation rate in the absence of a paramagnetic species and (1/Ti)p represents the additional paramagnetic contribution. In the absence of solute-solute interactions, the water relaxation rates are linearly dependent on the concentration of paramagnetic species, [M]. Relaxivity, rip, is defined as the slope of this dependence in units of mM-1 s-1 [Lauffer RB (1987) Chem Rev 87, 901] :
(1/Ti)obs= (1/Ti)d + rip[M] i=1,2
The value of rip is a function of temperature and magnetic field strength.
The large and fluctuating local magnetic field in the vicinity of a paramagnetic center provides this additional relaxation pathway for solvent nuclei. Since these fields fall off rapidly with distance, random translational diffusion of water molecules and the paramagnetic species as well as specific chemical interaction that bring the solvent molecules near the metal ion (e.g. within 5 Å) are important in transmitting the paramagnetic effect. Each type of chemical interaction can yield different relaxation efficiencies as governed by the distance and time scale of the interaction; the sum of these contributions and that due to translational diffusion gives the total relaxivity of the paramagnetic species. In general, the fluctuation of magnetic field can arise by (i) conformational changes within the molecule or detachment of a ligand bearing the nucleus of interest, e.g., a coordinated water molecule, (ii) reorientation of the molecules with respect to the external magnetic field, and (iii) longitudinal and transverse electron-spin relaxation.